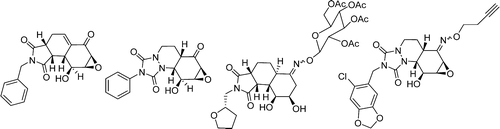
Stereocontrolled Synthesis of a Complex Library via Elaboration of Angular Epoxyquinol Scaffolds
Xiaoguang Lei, Nava Zaarur, Michael Y. Sherman, John A. Porco, Jr.
J. Org. Chem. 2005 , 70, 6474-6483
We have accomplished the synthesis of a complex chemical library via elaboration of angular epoxyquinol scaffolds with distinct skeletal frameworks. The key strategy involves highly stereocontrolled [4 + 2] Diels?Alder cycloadditions of chiral, nonracemic epoxyquinol dienes to generate the scaffolds. Further scaffold diversification involves hydrogenation, epimerization, dehydration, and condensation of the carbonyl group with alkoxyamine and carbazate building blocks. Further elaboration of the scaffolds also provided new skeletal frameworks using hydroxyl-directed Diels?Alder cycloaddition and reductive N?N bond cleavage. The overall process afforded 244 highly complex and functionalized compounds. Preliminary biological screening of the library uncovered six compounds which showed significant inhibition of Hsp 72 induction.