Biomimetic total synthesis of tricycloillicinone and mechanistic studies toward the rearrangement of prenyl phenyl ethers
Xiaoguang Lei, Mingji Dai, Zihao Hua, Samuel J. Danishefsky
Tetrahedron Lett. 2008 , 49 , 6383-6385
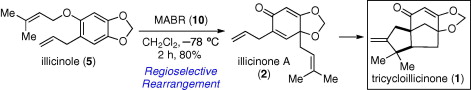
The Letter describes a short and biomimetic synthesis of tricycloillicinone, which was found to enhance the action of choline acetyltransferase (ChAT). The synthetic route has two critical reactions: bulky, oxygenophilic methylaluminum bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR) promoted rearrangement of prenyl phenyl ether and photochemical cyclization. Furthermore, experiments were designed to explore the process of MABR-promoted rearrangement. It was found that the stereochemistry of deuterium labeled prenyl group was only partially scrambled, which suggests that there may be two possible reaction pathways involved in this process. It also suggests that the direct migration of prenyl group to para-position under these conditions is slightly favored over the Claisen-Cope process. The highly efficient synthetic route also provides important new opportunities to explore the biological behavior of tricycloillicinone. ? 2008 Elsevier Ltd. All rights reserved.