Collective Synthesis of Lycopodium Alkaloids and Tautomer Locking Strategy for the Total Synthesis of ( – )-Lycojapodine A
Houhua Li, Xiaoming Wang, Benke Hong and Xiaoguang Lei*
J. Org. Chem. 2013, 78, 800-821 Featured and Cover Article
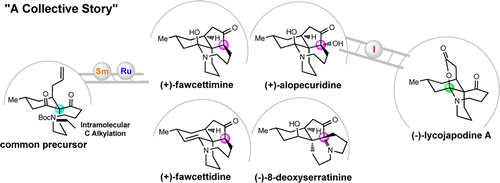
The collective total synthesis of?Lycopodium?alkaloids (+)-fawcettimine, (+)-fawcettidine, (+)-alopecuridine, (-)-lycojapodine A, and (-)-8-deoxyserratinine has been accomplished from a common precursor based on a highly concise route inspired by the proposed biosynthesis of the fawcettimine- and serratinine-type alkaloids. An intramolecular?C?-alkylation enabled efficient installation of the challenging spiro quaternary carbon center and the […]
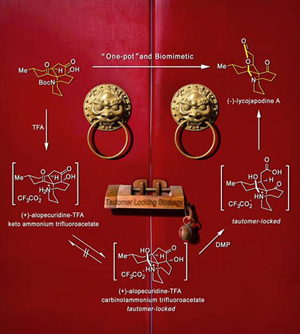
The collective total synthesis of?Lycopodium?alkaloids (+)-fawcettimine, (+)-fawcettidine, (+)-alopecuridine, (-)-lycojapodine A, and (-)-8-deoxyserratinine has been accomplished from a common precursor based on a highly concise route inspired by the proposed biosynthesis of the fawcettimine- and serratinine-type alkaloids. An intramolecular?C?-alkylation enabled efficient installation of the challenging spiro quaternary carbon center and the aza-cyclononane ring. The preparation of the tricyclic skeleton as well as the establishment of the correct relative stereochemistry of the oxa-quaternary center were achieved by hydroxyl-directed SmI 2-mediated pinacol couplings. An unprecedented tandem transannular?N?-alkylation and removal of a Boc group was discovered to realize a biosynthesis-inspired process to furnish the desired tetracyclic skeleton. Of particular note is the unique and crucial tautomer locking strategy employed to complete the enantioselective total synthesis of (-)-lycojapodine A. The central step in this synthesis is the late-stage hypervalent iodine oxidant (IBX or Dess–Martin periodinane)/TFA-mediated tandem process, which constructed the previously unknown carbinolamine lactone motif and enabled a biomimetic transformation to generate (-)-lycojapodine A in a single operation.