Chiral Boron Complex-Promoted Asymmetric Diels-Alder Cycloaddition and Its Application in Natural Product Synthesis
Li, X.; Han, J.; Jones, A.; Lei, X.*
J. Org. Chem. 2016, 81, 458-468
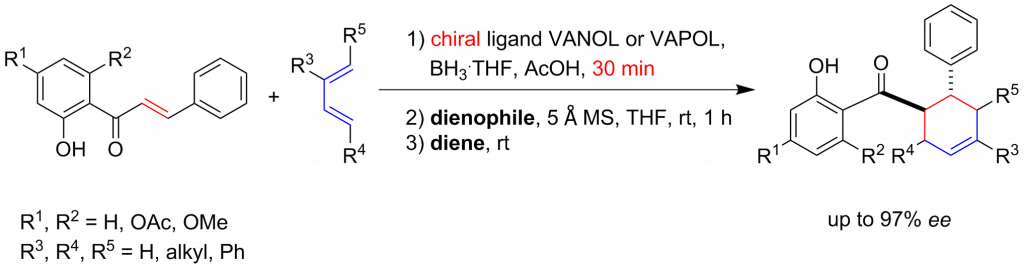
ABSTRACT An efficient method for the asymmetric Diels-Alder cycloaddition of 2’-hydroxychalcones with acyclic or cyclic dienes has been successfully developed. The Diels-Alder cycloaddition is mediated by a chiral boron complex with VANOL, affording the corresponding products in high yields and with excellent diastereo- and enantio-selectivities. This reaction enabled the enantioselective construction of cyclohexene skeletons crucial […]
ABSTRACT
An efficient method for the asymmetric Diels-Alder cycloaddition of 2’-hydroxychalcones with acyclic or cyclic dienes has been successfully developed. The Diels-Alder cycloaddition is mediated by a chiral boron complex with VANOL, affording the corresponding products in high yields and with excellent diastereo- and enantio-selectivities. This reaction enabled the enantioselective construction of cyclohexene skeletons crucial for the total synthesis of a number of Diels-Alder type natural products (-)-nicolaioidesin C, (-)-panduratine A, (-)-kuwanon I, (+)-kuwanon J, (-)-brosimones A and B.