Enantioselective Total Syntheses of Kuwanon X, Kuwanon Y and Kuwanol A
Lei Gao, Jianguang Han, and Xiaoguang Lei*
Org. Lett. 2016, 18, 360-363
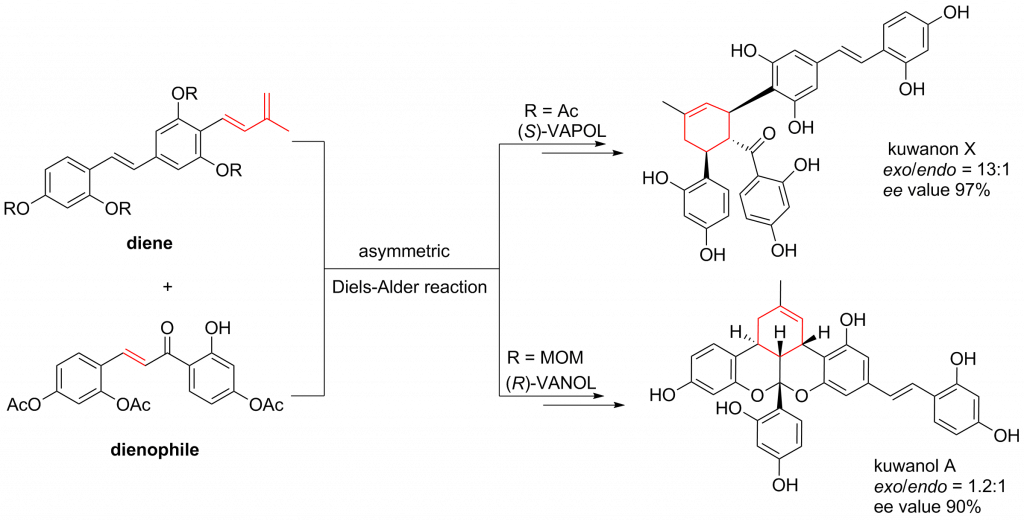
ABSTRACT: The first enantioselective total syntheses of?(?)-kuwanon X, (+)-kuwanon Y, and (+)-kuwanol A have been accomplished by using asymmetric Diels?Alder cycloaddition promoted by chiral VANOL or VAPOL/boron Lewis acid. The biosynthesis-inspired asymmetric Diels?Alder cycloaddition shows high exo selectivity (exo/endo = 13/1), which was unprecedented in the previous total syntheses of related prenylflavonoid Diels?Alder natural products. […]
ABSTRACT: The first enantioselective total syntheses of?(?)-kuwanon X, (+)-kuwanon Y, and (+)-kuwanol A have been accomplished by using asymmetric Diels?Alder cycloaddition promoted by chiral VANOL or VAPOL/boron Lewis acid. The biosynthesis-inspired asymmetric Diels?Alder cycloaddition shows high exo selectivity (exo/endo = 13/1), which was unprecedented in the previous total syntheses of related prenylflavonoid Diels?Alder natural products. An acid catalyzed intramolecular ketalization process enabled a biomimetic transformation to construct the polycyclic skeleton of kuwanol A efficiently.