Studies towards the Synthesis of the Functionalized C3-C14 Decalin Framework of Alchivemycin A
Ma, K.; Liao, D.; Yang, S.; Li, X.; Lei, X.*
Org. Chem. Front. 2016, 3, 251-258
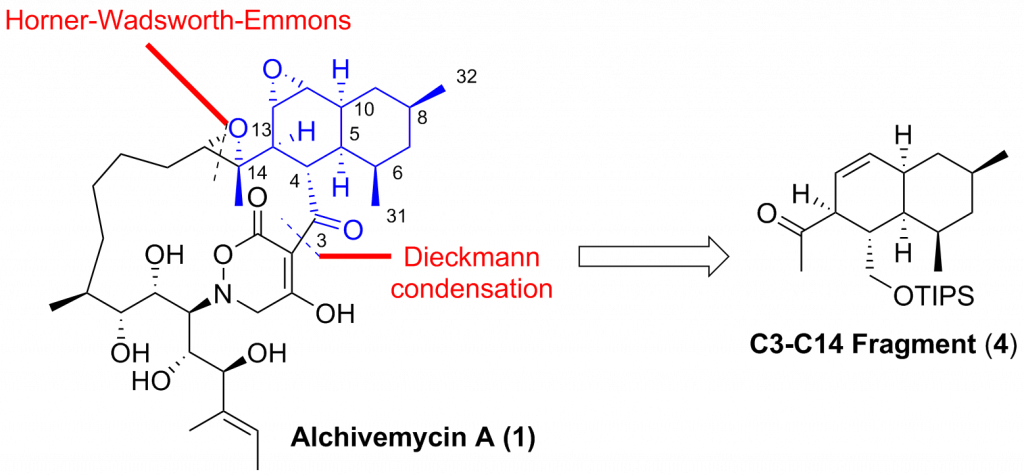
We report our synthetic studies towards the synthesis of the C3-C14 fragment of alchivemycin A. The synthesis featured an asymmetric alkylation with excellent diastereoselectivity and a one-pot Julia-Kocienski olefination with excellent E-selectivity. Intramolecular Diels-Alder reaction was employed to construct the highly functionalized cis-decalin framework. Interestingly, the stereochemical outcome was unexpected to generate two stereoisomers 20 […]
We report our synthetic studies towards the synthesis of the C3-C14 fragment of alchivemycin A. The synthesis featured an asymmetric alkylation with excellent diastereoselectivity and a one-pot Julia-Kocienski olefination with excellent E-selectivity. Intramolecular Diels-Alder reaction was employed to construct the highly functionalized cis-decalin framework. Interestingly, the stereochemical outcome was unexpected to generate two stereoisomers 20 and 21 instead of the desired cis-decalin 5. Detailed mechanism for this transformation was discussed. These synthetic endeavors have offered us a number of crucial insights for the synthesis of the complex natural product alchivemycin A.