Divergent total syntheses of ITHQ-type bis-bcarboline alkaloids by regio-selective formal aza-[4 + 2] cycloaddition and late-stage C–H functionalization
Qixuan Wang, Fusheng Guo, Jin Wang,Xiaoguang Lei
Chemical Science, 2023, 14(37), 10353-10359.
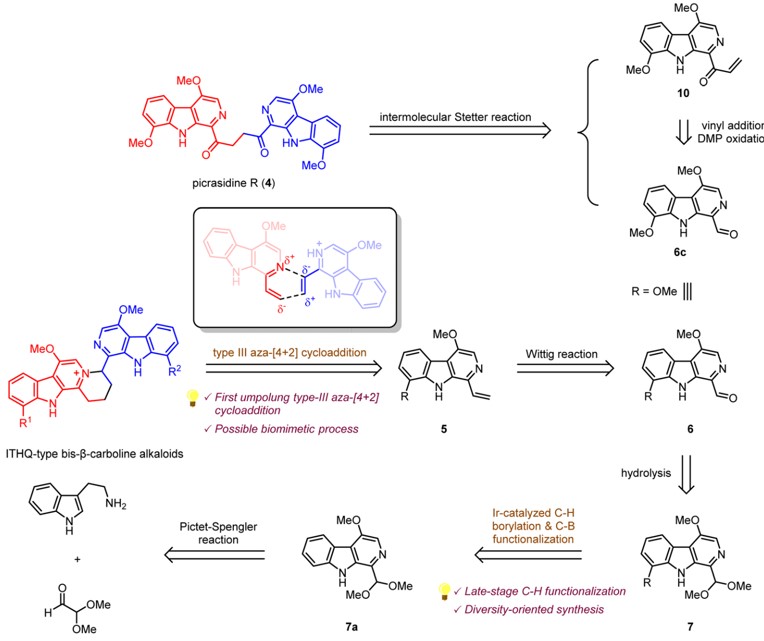
We herein report the first total syntheses of several bis-b-carboline alkaloids, picrasidines G, S, R, and T, and
natural product-like derivatives in a divergent manner. Picrasidines G, S, and T feature an indolotetrahydroquinolizinium (ITHQ) skeleton, while picrasidine R possesses a 1,4-diketone linker
between two b-carboline fragments. The synthesis of ITHQ-type bis-b-carboline alkaloids could be
directly achieved by a late-stage regio-selective aza-[4 + 2] cycloaddition of vinyl b-carboline alkaloids,
suggesting that this remarkable aza-[4 + 2] cycloaddition might be involved in the biosynthetic pathway.
Computational studies revealed that such aza-[4 + 2] cycloaddition is a stepwise process and explained
the unique regioselectivity (DDG = 3.77 kcal mol−1). Moreover, the successful application of iridiumcatalyzed C–H borylation on b-carboline substrates enabled the site-selective C-8 functionalization for
efficient synthesis and structural diversification of this family of natural products. Finally, concise synthesis of picrasidine R by the thiazolium-catalyzed Stetter reaction was also accomplished.
Wang, Q., Guo, F., Wang, J., & Lei, X. (2023). Divergent total syntheses of ITHQ-type bis-β-carboline alkaloids by regio-selective formal aza-[4+ 2] cycloaddition and late-stage C–H functionalization. Chemical Science, 14(37), 10353-10359.
We herein report the first total syntheses of several bis-b-carboline alkaloids, picrasidines G, S, R, and T, and natural product-like derivatives in a divergent manner. Picrasidines G, S, and T feature an indolotetrahydroquinolizinium (ITHQ) skeleton, while picrasidine R possesses a 1,4-diketone linker between two b-carboline fragments. The synthesis of ITHQ-type bis-b-carboline alkaloids could be directly achieved by a late-stage regio-selective aza-[4 + 2] cycloaddition of vinyl b-carboline alkaloids, suggesting that this remarkable aza-[4 + 2] cycloaddition might be involved in the biosynthetic pathway. Computational studies revealed that such aza-[4 + 2] cycloaddition is a stepwise process and explained the unique regioselectivity (DDG = 3.77 kcal mol-1). Moreover, the successful application of iridiumcatalyzed C–H borylation on b-carboline substrates enabled the site-selective C-8 functionalization for efficient synthesis and structural diversification of this family of natural products. Finally, concise synthesis of picrasidine R by the thiazolium-catalyzed Stetter reaction was also accomplished.