Chemoenzymatic total synthesis of alchivemycin A
Haoran Dong, Nianxin Guo , Dachao Hu, Benke Hong, Daohong Liao, Hong Jie Zhu, Zhang Yuan Yan, Hui Ming Ge & Xiaoguang Lei
Nature Synthesis. Published online: 25 June 2024.
doi: 10.1038/s44160-024-00577-7
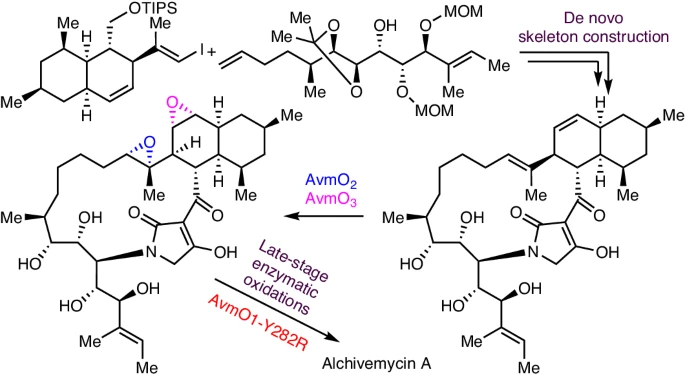
Alchivemycin A belongs to a unique class of polyketide natural products isolated from plant-derived actinomycete Streptomyces. It shows potent antibacterial activity and anti-tumour activity. However, its inherent structural complexity and high oxidation state, especially the 2H-tetrahydro-4,6-dioxo-1,2-oxazine (TDO) ring system, present synthetic challenges. Here we report the total synthesis of alchivemycin A using a chemoenzymatic approach that combines de novo skeleton construction and late-stage enzymatic oxidation reactions. The convergent synthesis of the highly functionalized unnatural tetramic acid-bearing intermediate is achieved by boron-alkyl Suzuki−Miyaura cross-coupling, macrolactamization and Lacey–Dieckmann condensation reactions. Efficient enzymatic epoxidations using the redox enzymes AvmO3 and AvmO2 allow rapid access to the desired diepoxide product regio- and stereoselectively. Subsequently, a flavin adenine dinucleotide-dependent enzyme AvmO1 variant optimized via rational protein engineering, AvmO1-Y282R, was used to convert the tetramic acid ring into the TDO ring through a Baeyer–Villiger-type transformation, completing the chemoenzymatic synthesis of alchivemycin A. This work paves the way to further explore the biological functions of alchivemycin A and highlights the utility of chemoenzymatic strategies to tackle synthetic challenges in complex molecule synthesis.
Alchivemycin A belongs to a unique class of polyketide natural products isolated from plant-derived actinomycete Streptomyces. It shows potent antibacterial activity and anti-tumour activity. However, its inherent structural complexity and high oxidation state, especially the 2H-tetrahydro-4,6-dioxo-1,2-oxazine (TDO) ring system, present synthetic challenges. Here we report the total synthesis of alchivemycin A using a chemoenzymatic approach that combines de novo skeleton construction and late-stage enzymatic oxidation reactions. The convergent synthesis of the highly functionalized unnatural tetramic acid-bearing intermediate is achieved by boron-alkyl Suzuki−Miyaura cross-coupling, macrolactamization and Lacey–Dieckmann condensation reactions. Efficient enzymatic epoxidations using the redox enzymes AvmO3 and AvmO2 allow rapid access to the desired diepoxide product regio- and stereoselectively. Subsequently, a flavin adenine dinucleotide-dependent enzyme AvmO1 variant optimized via rational protein engineering, AvmO1-Y282R, was used to convert the tetramic acid ring into the TDO ring through a Baeyer–Villiger-type transformation, completing the chemoenzymatic synthesis of alchivemycin A. This work paves the way to further explore the biological functions of alchivemycin A and highlights the utility of chemoenzymatic strategies to tackle synthetic challenges in complex molecule synthesis.