Hunting for the Intermolecular Diels−Alderase
Lei Gao, Qi Ding, Xiaoguang Lei*
Accounts of Chemical Research, doi:10.1021/acs.accounts.4c00315
The Diels–Alder reaction is well known as a concerted [4 + 2] cycloaddition governed by the Woodward–Hoffmann rules. Since Prof. Otto Diels and his student Kurt Alder initially reported the intermolecular [4 + 2] cycloaddition between cyclopentadiene and quinone in 1928, it has been recognized as one of the most powerful chemical transformations to build C–C bonds and construct cyclic structures. This named reaction has been widely used in synthesizing natural products and drug molecules. Driven by the synthetic importance of the Diels–Alder reaction, identifying the enzyme that stereoselectively catalyzes the Diels–Alder reaction has become an intriguing research area in natural product biosynthesis and biocatalysis. With significant progress in sequencing and bioinformatics, dozens of Diels–Alderases have been characterized in microbial natural product biosynthesis. However, few are evolutionally dedicated to catalyzing an intermolecular Diels–Alder reaction with a concerted mechanism.
The Diels–Alder reaction is well known as a concerted [4 + 2] cycloaddition governed by the Woodward–Hoffmann rules. Since Prof. Otto Diels and his student Kurt Alder initially reported the intermolecular [4 + 2] cycloaddition between cyclopentadiene and quinone in 1928, it has been recognized as one of the most powerful chemical transformations to build C–C bonds and construct cyclic structures. This named reaction has been widely used in synthesizing natural products and drug molecules. Driven by the synthetic importance of the Diels–Alder reaction, identifying the enzyme that stereoselectively catalyzes the Diels–Alder reaction has become an intriguing research area in natural product biosynthesis and biocatalysis. With significant progress in sequencing and bioinformatics, dozens of Diels–Alderases have been characterized in microbial natural product biosynthesis. However, few are evolutionally dedicated to catalyzing an intermolecular Diels–Alder reaction with a concerted mechanism.
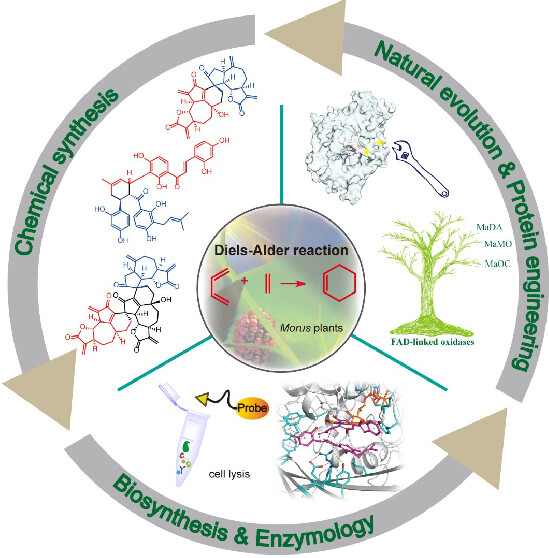
This Account summarizes our endeavors to hunt for the naturally occurring intermolecular Diels–Alderase from plants. Our research journey started from the biomimetic syntheses of D–A-type terpenoids and flavonoids, showing that plants use both nonenzymatic and enzymatic intermolecular [4 + 2] cycloadditions to create complex molecules. Inspired by the biomimetic syntheses, we identify an intermolecular Diels–Alderase hidden in the biosynthetic pathway of mulberry Diels–Alder-type cycloadducts using a biosynthetic intermediate probe-based target identification strategy. This enzyme, MaDA, is an endo-selective Diels–Alderase and is then functionally characterized as a standalone intermolecular Diels–Alderase with a concerted but asynchronous mechanism. We also discover the exo-selective intermolecular Diels–Alderases in Morus plants. Both the endo- and exo-selective Diels–Alderases feature a broad substrate scope, but their mechanisms for controlling the endo/exo pathway are different. These unique intermolecular Diels–Alderases phylogenetically form a subgroup of FAD-dependent enzymes that can be found only in moraceous plants, explaining why this type of [4 + 2] cycloadduct is unique to moraceous plants. Further studies of the evolutionary mechanism reveal that an FAD-dependent oxidocyclase could acquire the Diels–Alderase activity via four critical amino acid mutations and then gradually lose its original oxidative activity to become a standalone Diels–Alderase during the natural evolution. Based on these insights, we designed new Diels–Alderases and achieved the diversity-oriented chemoenzymatic synthesis of D–A products using either naturally occurring or engineered Diels–Alderases.
Overall, this Account describes our decade-long efforts to discover the intermolecular Diels–Alderases in Morus plants, particularly highlighting the importance of biomimetic synthesis and chemical proteomics in discovering new intermolecular Diels–Alderases from plants. Meanwhile, this Account also covers the evolutionary and catalytic mechanism study of intermolecular Diels–Alderases that may provide new insights into how to discover and design new Diels–Alderases as powerful biocatalysts for organic synthesis.