Research News, Sept 10th, 2009
Hydride-induced Novel Cyclization of Dienedinitriles Leading to Multi-functionalized Cyclopentadienes
Hui-jun Zhang, Tianhao Meng, Bernard Demerseman, Christian Bruneau* and Zhenfeng Xi*
Org. Lett. 2009, ASAP online.
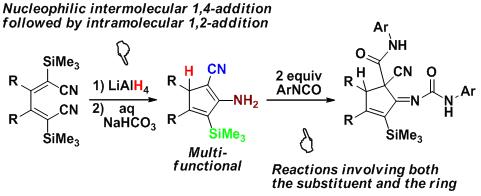
By treatment with LiAlH4, 1,4-dicyano-1,4-bis(trimethylsilyl)-1,3-dienes underwent a novel hydride-induced nucleophilic intermolecular 1,4-addition to the a, b-unsaturated nitrile moiety, followed by an immediate nucleophilic intramolecular 1,2-addition to the remaining CN group, to afford multiply functionalized cyclopentadienes in high isolated yields. These multi-functional (-CN, -NH2, -SiMe3, -H) cyclopentadienes are of a rich and interesting reaction chemistry as preliminarily demonstrated by their reaction with ArNCO involving both the substituent and the ring.
