Research News, Sept 10th, 2009
Skeletal Rearrangement of All-carbon Spiro Skeletons
Mediated by Lewis Acid
Fei Zhao, Chao Wang, Lantao Liu, Wen-Xiong Zhang, and Zhenfeng Xi*
Chem. Commun. 2009, ASAP online.
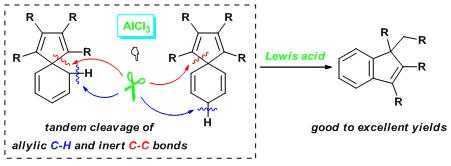
Mediated by AlCl3, substituted spiro[4.5]deca-tetraenesunderwent novel and selective skeletal rearrangement to generate indene derivatives in high to excellent yields.Selective cleavage of C-C and C-H bond is of great importance both fundamentally and practically. Sequential cleavage of both C–C and C–H bonds in all-carbon cyclic compounds spiro[4.5]deca-tetraenes was achieved to generate indene derivates via a novel AlCl3-mediated process.
This work has the following two features. (1) First example on the cleavage of C-C and C-H bond of all-carbon spiro compounds; (2) A new protocol on cleavage of C-C and C-H bond.
