Monomethylation and -protonation of Lutetium Dinitrogen Complex
Xiao Chen, Gao-Xiang Wang, Ze-Jie Lv, Junnian Wei,* Zhenfeng Xi
J. Am. Chem. Soc., 2024. DOI: 10.1021/jacs.4c05492.
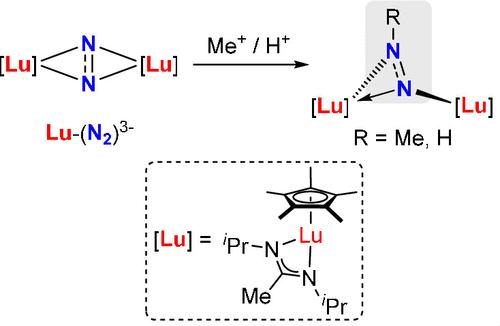
Due to the highly chemically inert nature, direct activation and transformation of dinitrogen are challenging. Here, we disclose the synthesis, isolation, and derivatization of (N2)3– supported by lutetium complex. Initially, a (N2)3– radical, in [{(C5Me5){MeC(NiPr)2}Lu}2(μ2-η2:η2-N2)][K(crypt)] (crypt = 2,2,2-cryptand) complex, was generated through the reduction of neutral lutetium dinitrogen complex [{(C5Me5){MeC(NiPr)2}Lu}2(μ2-η2:η2-N2)] with potassium metal. Subsequently, the reaction of (N2)3– complex with methyl triflate (or triflic acid) led to the formation of an N–C (or N–H) bond, yielding the corresponding [{(C5Me5){MeC(NiPr)2}Lu}2(NN-R)(OTf)][K(crypt)] (R = Me, H, OTf = CF3SO3) as the product. Both electron paramagnetic resonance spectroscopy and density functional theory analyses support the radical character of the NN-Me unit. The Lu–N bonds in the (NN-Me)•2– radical complex are predominantly ionic, with 77% of the unpaired electron localized on the (NN-Me) fragment. Moreover, the geometry of the pure organic radical (NN-Me)•2–, optimized by double-hybrid density functional theory, closely matches that of the (NN-Me)•2– lutetium complex.