Dinitrogen Reduction Chemistry with Scandium Provides a Complex with Two Side-on (N=N)2− Ligands Bound to One Metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2
Joshua D Queen, Ahmadreza Rajabi, Quinn E. Goudzwaard, Qiong Yuan, Dang Khoa Nguyen, Joseph W Ziller, Filipp Furche*, Zhenfeng Xi*, William J Evans*
Chem. Sci., 2024. DOI: 10.1039/D4SC03977G.
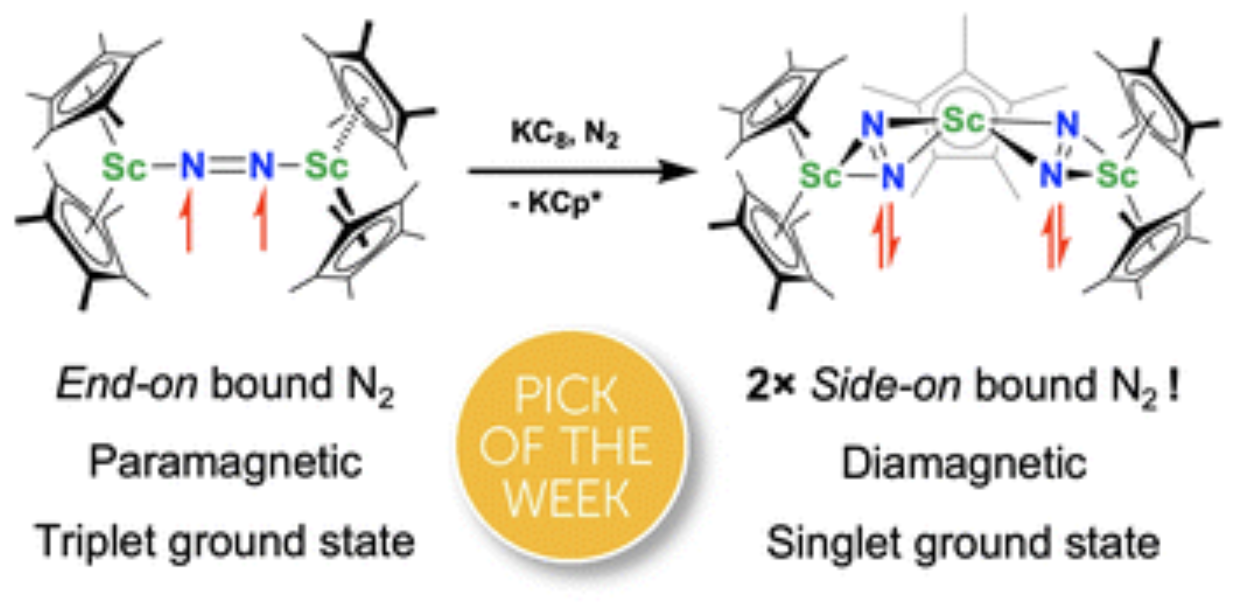
Although there are few reduced dinitrogen complexes of scandium, this metal has revealed a new structural type in reductive dinitrogen chemistry by reduction of bis(pentamethylcyclopentadienyl) scandium halides under N2. Reduction of Cp*2ScI (Cp* = C5Me5) with potassium graphite (KC8) under dinitrogen generates the dark blue paramagnetic complex (Cp*2Sc)2(μ-η1:η1-N2), 1. This end-on bridging (N=N)2− complex is a diradical with a magnetic moment of 2.8µB. Upon further reduction of 1 with KC8, the orange diamagnetic trimetallic complex Cp*Sc[(μ-η2:η2-N2)ScCp*2], 2, is obtained. This complex has an unprecedented structure in which two side-on bridging (N=N)2− ligands are bound to the central (Cp*Sc)2+ moiety. Complex 2 can also be obtained directly from reduction of or Cp*2ScI a mixture of Cp*2ScI and Cp*2ScCl(THF) with KC8. The reaction of Cp*2ScI with KC8 in the presence of 18-crown-6 or 2.2.2-cryptand affords 2 along with small amounts of Cp*2Sc(μ-η2:η2-N2)ScCp*I(THF), 3, which is green at room temperature and purple at low temperature and displays a mixture of side-on and end-on bridging isomers in the crystal structure collected at −180 °C. Density functional theory (DFT) calculations are consistent with a triplet ground state for the end-on complex 1 and singlet ground states for the side-on complexes 2 and 3.