Construction of N−E bonds via Lewis acid-promoted functionalization of chromium-dinitrogen complexes
Zhu-Bao Yin, Gao-Xiang Wang, Xuechao Yan, Junnian Wei*, Zhenfeng Xi
Nat. Commun., 2025. DOI: 10.1038/s41467-025-55998-5.
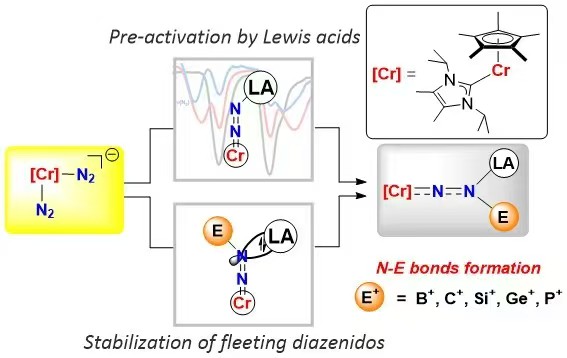
Direct conversion of dinitrogen (N2) into N-containing compounds beyond ammonia under ambient conditions remains a longstanding challenge. Herein, we present a Lewis acid-promoted strategy for diverse nitrogen-element bonds formation from N2 using chromium dinitrogen complex [Cp*(IiPr2Me2)Cr(N2)2]K (1). With the help of Lewis acids AlMe3 and BF3, we successfully trap a series of fleeting diazenido intermediates and synthesize value-added compounds containing N−B, N−Ge, and N−P bonds with 3d metals, offering a method for isolating unstable intermediates. Furthermore, the formation of N−C bonds is realized under more accessible conditions that avoid undesired side reactions. DFT calculations reveal that Lewis acids enhance the participation of dinitrogen units in the frontier orbitals, thereby promoting electrophilic functionalization. Moreover, Lewis acid replacement and a base-induced end-on to side-on switch of [NNMe] unit in [(Cp*(IiPr2Me2)CrNN(BEt3)(Me)] (8) are achieved.