Prof. Lei recently received “2015 The Distinguished Lectureship Award” by Chemical Society of Japan (CSJ). He was invited to attend the CSJ annual meeting in Chiba, March 26-29 and present a keynote lecture entitled “Dissectin Cell Death with Complex Natural Products”. This prestigious award recognizes Prof. Lei’s remarkable contributions to the chemical biology studies of […]
Congratulations to Benke, Houhua, Jinbao and Jing for their latest total synthesis published on ACIE! Angew. Chem. Int. Ed. 2014 , DOI: 10.1002/anie.201409503 Abstract: Utilizing a late-stage enamine bromofunctionlization strategy, we have accomplished a 12-step total synthesis of (-)-huperzine Q as well as the first total syntheses of (+)-lycopladines B and C. We also report […]
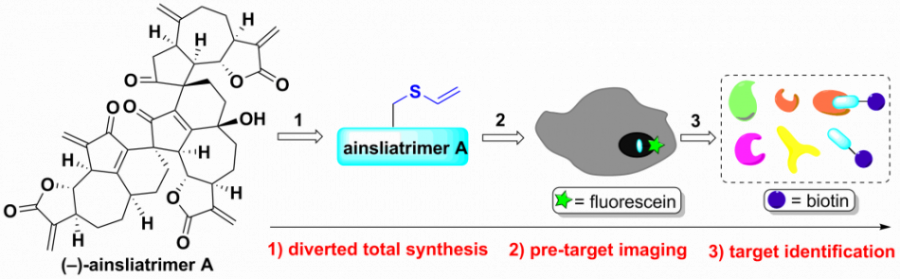
Natural products have played a crucial role in the development of new therapeutic agents. However, the target identification for natural products is still challenging due to the structural complexity and the lack of efficient biochemical methods. In the previous studies, we have accomplished the collective total syntheses of a number of structurally complex and bioactive […]
Our graduate students Mr. Jianguang Han, Mr. Weilong Liu, Mr. Benke Hong, Ms. Ting Dong, and Mr. Jing Zhang won the “2014 NIBS Excellent Student Award” for their outstanding performance in scientific research.?Big congrats?to all of them!!! Our previous awardees are: 2011 Mr. Chao Li; 2012 Mr. Xiaoming Wang; 2013 Mr. Daohong Liao and Mr. […]
Prof. Lei received the Chinese Chemical Society Young Investigator Award in the National Chemistry Symposium held at Peking University on August 4-8. This is the most prestigious award ?for the young chemist?in China?under the age of 35 who has made outstanding accomplishments in the research of chemistry. During the past 5 years, Prof. Lei and […]
Our paper entitled “Diversity-oriented synthesis of Lycopodium alkaloids inspired by the hidden functional group pairing pattern” has been published on Nature Communications. In this study, we report a new concept of Functional Group Pairing Pattern Recognition (FGPPR) to facilitate both target-oriented synthesis of complex natural product and diversity-oriented synthesis of natural product-like scaffolds in parallel. […]
Our??paper entitled “Enantioselective Biomimetic Total Syntheses of Kuwanons I and J and?Brosimones A and B” has been published on Angew. Chem. Int. Ed. 2014, DOI:anie.201404499 Abstract: The first enantioselective total syntheses of prenylflavonoid Diels-Alder natural products (-)-kuwanon I, (+)-kuwanon J, (-)-brosimone A and (-)-brosimone B have been accomplished based on a concise synthetic strategy. Key […]
-

-
-
-
-

-
-
-
-
-
Prof. Lei received 2015 The Distinguished Lectureship Award by CSJ
Prof. Lei recently received “2015 The Distinguished Lectureship Award” by Chemical Society of Japan (CSJ). He was invited to attend the CSJ annual meeting in Chiba, March 26-29 and present a keynote lecture entitled “Dissectin Cell Death with Complex Natural Products”. This prestigious award recognizes Prof. Lei’s remarkable contributions to the chemical biology studies of […]
Celebration party, 2015/1/31
We have a celebration party for Weilong and Jing’s significant breakthroughs in complex natural product syntheses!
Prof. Lei received Outstanding Young Talent Award by Beijing Government, 2014
Prof. Lei received the Roche Young Investigator Award, 2014
Total Syntheses of (-)-Huperzine Q, (+)-Lycopladines B and C
Congratulations to Benke, Houhua, Jinbao and Jing for their latest total synthesis published on ACIE! Angew. Chem. Int. Ed. 2014 , DOI: 10.1002/anie.201409503 Abstract: Utilizing a late-stage enamine bromofunctionlization strategy, we have accomplished a 12-step total synthesis of (-)-huperzine Q as well as the first total syntheses of (+)-lycopladines B and C. We also report […]
We have discovered a new mode of anticancer action of the natural product ainsliatrimer A
Natural products have played a crucial role in the development of new therapeutic agents. However, the target identification for natural products is still challenging due to the structural complexity and the lack of efficient biochemical methods. In the previous studies, we have accomplished the collective total syntheses of a number of structurally complex and bioactive […]
Jianguang, Weilong, Benke, Ting, and Jing won the 2014 NIBS Excellent Student Award!
Our graduate students Mr. Jianguang Han, Mr. Weilong Liu, Mr. Benke Hong, Ms. Ting Dong, and Mr. Jing Zhang won the “2014 NIBS Excellent Student Award” for their outstanding performance in scientific research.?Big congrats?to all of them!!! Our previous awardees are: 2011 Mr. Chao Li; 2012 Mr. Xiaoming Wang; 2013 Mr. Daohong Liao and Mr. […]
Prof. Lei has received the Chinese Chemical Society Young Investigator Award, Congratulations!
Prof. Lei received the Chinese Chemical Society Young Investigator Award in the National Chemistry Symposium held at Peking University on August 4-8. This is the most prestigious award ?for the young chemist?in China?under the age of 35 who has made outstanding accomplishments in the research of chemistry. During the past 5 years, Prof. Lei and […]
Jing’s work has been published on Nature Communications, Congratulations!
Our paper entitled “Diversity-oriented synthesis of Lycopodium alkaloids inspired by the hidden functional group pairing pattern” has been published on Nature Communications. In this study, we report a new concept of Functional Group Pairing Pattern Recognition (FGPPR) to facilitate both target-oriented synthesis of complex natural product and diversity-oriented synthesis of natural product-like scaffolds in parallel. […]
Our latest natural product total synthesis on ACIE!
Our??paper entitled “Enantioselective Biomimetic Total Syntheses of Kuwanons I and J and?Brosimones A and B” has been published on Angew. Chem. Int. Ed. 2014, DOI:anie.201404499 Abstract: The first enantioselective total syntheses of prenylflavonoid Diels-Alder natural products (-)-kuwanon I, (+)-kuwanon J, (-)-brosimone A and (-)-brosimone B have been accomplished based on a concise synthetic strategy. Key […]